Quality applied to automation engineering and software development is mandatory for the pharmaceutical, medical and food industries.
We support your organization in order to implement all quality requirements on your systems.
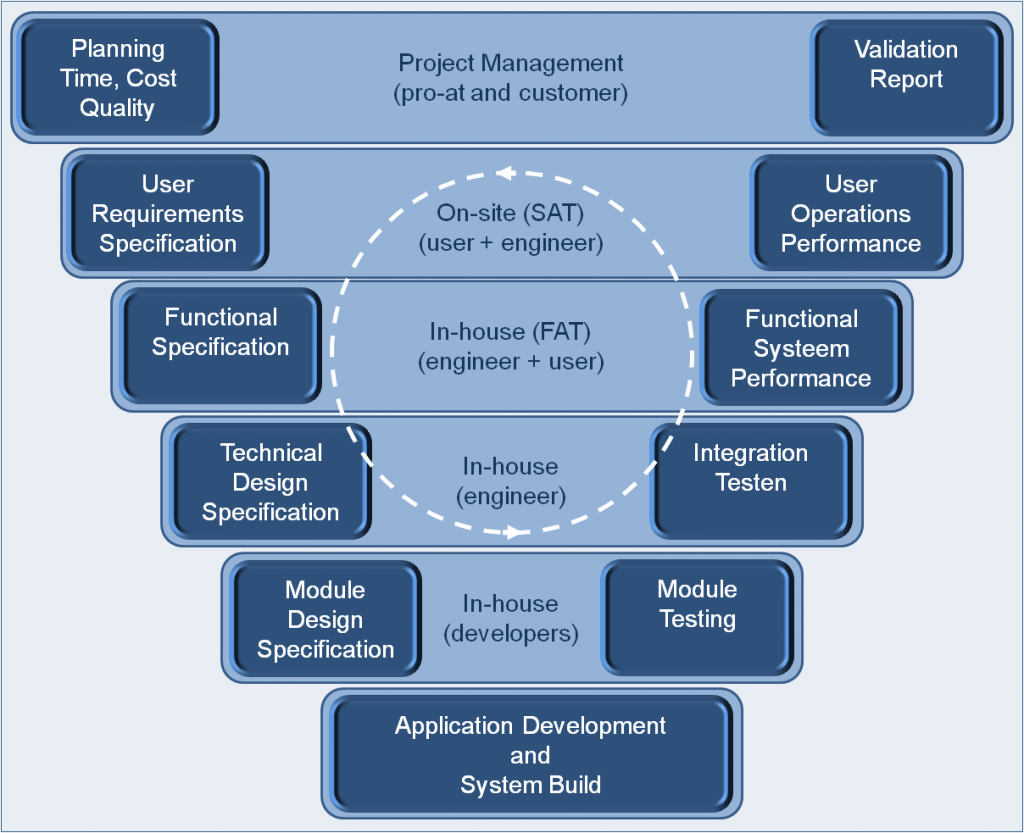
We support you in the following topics :
- Automation quality life cycle
- GAMP methodology
- Computerized System Validation (CSV)
- Requirements for Electronic Records & Electronic Signatures (21CFR Part 11)




